What is Reversed Carnot Cycle?
Reversed Carnot Cycle is a type of Refrigeration Cycle that is exactly opposite (or reverse) to the Carnot Cycle. The processes that involved in the Carnot and Reversed Carnot Cycle are same but the procedure and working sequence is different and quite opposite each other.
The Carnot Cycle is used to convert the convert the heat into the mechanical work whereas; the Reversed Carnot Cycle (or refrigeration system) is used to absorb the heat from the system and rejects to the surroundings (or environment) to maintain the system cool (which we called refrigeration effect).
Also Read
Carnot Theorem, Carnot Cycle, Processes, Efficiency, Limitations, and Applications
What can you learn from this post?
- Processes In Reversed Carnot Cycle
- Cop Of Reversed Carnot Cycle
- Limitations Of Reversed Carnot Cycle
- Applications Of Reversed Carnot Cycle
- Conclusion
Processes in Reversed Carnot Cycle:
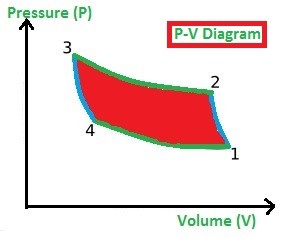
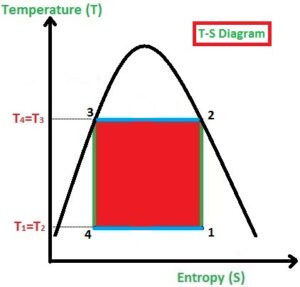
This process is used to build the refrigeration system so the working process is all about achieving the cooling environment in the system or space. As like Carnot Cycle, the Reversed Carnot Cycle too consists of four processes. They are
- Isentropic or Adiabatic Process (1-2)
- Isothermal Compression Process (2-3)
- Isentropic or Adiabatic Expansion Process (3-4)
- Isothermal Expansion Process (4-1)
Isentropic or Adiabatic Compression Process (1-2):
In the Isentropic (or adiabatic) Compression process (1-2), the gas is compressed. Due to the compression of gas, the pressure is increased highly and volume will be decreased slightly (observe the P-V Diagram) and at the same time, the Entropy (S) remains constant (which we called as an isentropic process).
Isothermal Compression Process (2-3):
In the Isothermal Compression process (2-3), the heat is added at a constant temperature. Due to the prolonged compression of gas, the pressure is increased slightly and volume will be decreased highly (observe the P-V Diagram) and at the same time, the Temperature (T) remains constant. (Observe the T-S diagram)
Isentropic or Adiabatic Expansion Process (3-4):
In the Isentropic Expansion process (3-4), the pressure is decreased highly and volume will be increased slightly (observe the P-V Diagram) and at the same time, the Entropy (T) remains constant. (Observe the T-S diagram)
Isothermal Expansion Process (4-1):
In the Isothermal Expansion process (4-1), the heat is rejected by the gas to the surroundings at a constant temperature, the pressure is decreased slightly and volume will be increased highly (observe the P-V Diagram) and at the same time, the Temperature (T) remains constant (Observe the T-S diagram).
Also Read
What Is Four Stroke Engine, Working Of Four Stroke Engine, Advantages?
What Is Two-Stroke Engine, Working Of Two Stroke Engine – Parts, And Advantages?
COP Of Reversed Carnot Cycle:
COP stands for Coefficient Of Performance that is used to estimate the performance of the Cycle. As this is the Refrigeration Cycle, the COP of the cycle is estimated as the ratio of the amount of heat absorbed by the source to the net-work done.
The net work done on the system can be explained as the difference between the heat absorbed by the source and the heat rejected by the source (Here, the source will be the gas that is used in the refrigeration process).
So, the mathematical expression of the COP of the Reversed Carnot Cycle is

Limitations Of Reversed Carnot Cycle:
The limitations are the drawbacks of any system or cycle. So, even Reversed Carnot Cycle does consist of Limitations in its way. Let us see the limitations of Reversed Carnot Cycle
- The application of isentropic and isothermal processes back to back is impossible because, in the isentropic process, the pressure increases fastly which requires a high speed, and in the isothermal process, the pressure increase slowly which requires low speed so, achieving high and slow processes back to back is impossible in real life.
- Another side, the addition, and rejection of heat at constant temperature are impossible because whenever the heat is added to any source, the temperature will be increased usually which is impossible to restrict.
So, according to the limitations of Reversed Carnot Cycle, we can conclude that the construction of Reversed Carnot Cycle is impossible in real life.
Applications Of Reversed Carnot Cycle:
The Carnot cycle and Reversed Carnot Cycles are Ideal cycles which means the construction of such cycles in real life is impossible. So, we can say that the applications of both cycles do not exist. But we can say that the Reversed Carnot Cycle is an ideal cycle for the Refrigeration System and Effect.
Conclusion:
The Reversed Carnot Cycle construction is impossible but it is being an ideal cycle for the refrigeration system, the process looks the same like Carnot Cycle but the sequence of applying each process is reversed.
We can say that the heat engine is a system that work done by the system but when it comes to the refrigeration system (or Reversed Carnot Cycle) the work is done on the system. As the cycle is ideal, the coefficient of performance of the refrigeration is also high and it is impossible to achieve by any other process.