What Is Carnot Theorem?
Carnot Theorem is a theorem of heat engine which states that “the engine which works between two reservoirs (heat and cold reservoirs) will possess less efficient than the Reversible Heat Engine or Carnot Heat Engine or Carnot Cycle. It is the concept we study in the Thermodynamics subject.
Also Read:
What can you learn from this post?
- Carnot Cycle
- Carnot Cycle Process
- Efficiency Of Carnot Cycle
- Limitations Of Carnot Cycle
- Applications Of Carnot Cycle
- Conclusion
Carnot Cycle:
Carnot Cycle is an Ideal (Unique and Impossible) reversible heat engine cycle that develops the power to do some work. The whole working principle of the Carnot Cycle will be the conversion of heat energy into useful work. As this is a heat engine, we can say that the Carnot Cycle a type of thermodynamics cycle.
Also Read:
- Two Stroke Engine – Working Of Two Stroke Engine, And Its Advantages
- Four Stroke Engine – Working Of Four Stroke Engine, And Its Avantages
- Advantages Of Four Stroke Engine Over Two-Stroke Engine
As usual, the Carnot Cycle does consist of parts working processes and parts. Let us discuss the parts of the Carnot Cycle and Carnot Cycle Process.
Carnot Cycle Process:
The Carnot Cycle consists of Four working process that involves two isothermal and two adiabatic processes. Let us read what actually these isothermal and adiabatic mean?
What Is Isothermal Process?
Isothermal Process is an ideal process where the temperature will be constant throughout the process or working. Even though the temperature is constant, we can expect a change of phase (like conversion of water into the vapour).
What is adiabatic Process?
Adiabatic Process is another process where the temperature addition and rejection will not take place in a particular action or process. In the adiabatic process, due to the restriction of temperature addition and rejection, there will be no phase change occurs.

The Carnot Cycle is explained with the help of Piston, and Cylinder equipment. As the heat added to the cylinder, the gas will expand which results in the movement of the piston, and at the same time whenever the heat is rejected from the cylinder, the gas will get compressed by the piston.
This addition of heat, expansion of gas, rejection of heat, and compression of gas is systematically processed into Four Processes and they are as follows

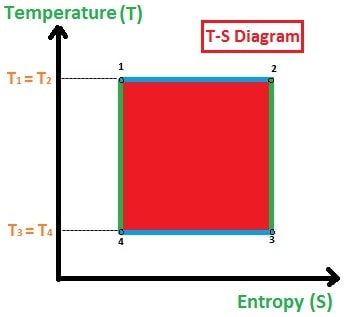
1. Isothermal Expansion Process:
In the isothermal expansion process (1-2), the heat is absorbed by the gas qin (heat in) constantly from the heat reservoir. So, the temperature will be taken as T1.
In this process, the pressure will be a little bit decreased and the volume of the cylinder will be increased (observe P-V diagram). The temperature remains constant and entropy will increase (observe T-S diagram).
2. Adiabatic Expansion Process:
In the adiabatic expansion process (2-3), the gas will not absorb and reject the heat instead the gas will expand more as compared to the isothermal expansion and pushes the piston upward direction.
In this process, the pressure will suddenly decrease and volume will be slightly increased (observe P-V diagram). The temperature will decrease and entropy will be constant (observe T-S diagram).
3. Isothermal Compression Process:
In the isothermal compression process (3-4), the heat is rejected by the gas qout and the temperature remains constant. Now, the piston will compress the gas.
Due to this compression, the pressure is increased slightly and volume will be decreased much (observe P-V diagram). The temperature remains constant and entropy will decrease (observe T-S diagram).
4. Adiabatic Compression Process:
In the adiabatic compression process (4-1), the temperature neither absorbed nor rejected by the gas, and the compression will continue until the piston reaches its original state.
Due to the compression, the pressure increase suddenly and volume decreases slightly (observe P-V diagram). The temperature will be increased and entropy will be constant (observe T-S diagram).
Efficiency Of Carnot Cycle:
The efficiency is the measuring term that how well a device or system working and how well the output of a device is. As said earlier, the Carnot Cycle is an ideal cycle that gives high and maximum efficiency.
The efficiency of the Carnot Cycle is defined as the ratio of net work done by the cycle to the heat absorbed by the cycle. Whereas; we can say that the work done by the cycle is nothing but the difference between the heat absorbed by the gas QA and the heat rejected by the gas QR. So, the mathematical expression of efficiency of the Carnot Cycle is

We can write the above formula in terms of Temperatures. The maximum efficiency that Carnot Cycle can develop is given as the ratio of temperature differences of two reservoirs to the higher temperature reservoir. Let us assume the temperature of higher reservoir as T1 and the temperature of lower reservoir as T2 then, the Carnot Cycle efficiency will be

Limitations Of Carnot Cycle:
The limitations of Carnot Cycle are as follows
- The Carnot Cycle is an ideal cycle which means that did not exist and impossible to construct so, it is just a theoretical concept.
- The isothermal process says that the temperature is constant but the Carnot Cycle explains there will be heat addition in the isothermal expansion process which is not possible.
- The Carnot Cycle is used to study the heat engine and not extend to other types of devices.
- In the practical engine, the heat loss will be possible wherein the Carnot Cycle it is not mentioned which results in the maximum efficiency (which is not possible).
Applications Of Carnot Cycle:
The Carnot Cycle is an ideal cycle so, it is not possible to construct and develop such models in real life. So, we can say that there are no applications of the Carnot Cycle and Carnot Engine in real life. But the Carnot Cycle and process are developed for heat engines.
Conclusion:
Carnot Cycle is purely an ideal cycle that cannot be constructed and developed in real life. It is a referring cycle that every student and engineer should aware of. The Carnot Cycle is almost over-written with all the basic concepts like isothermal process and adiabatic process to justify his concepts.
Carnot said the heat is absorbed at a constant temperature which is not possible in real life. In real life, there will be loss of heat while adding and rejection whereas; it is impossible to convert the whole heat into useful work.