What is Thermal Engineering?
Thermal Engineering is one of the subjects in Mechanical Engineering and even in some other branches that deal with the heat energy and its laws, Air Cycles and their Applications, Energy producing devices, etc. The first and most important section or sub-division that we need to learn in Thermal Engineering is Thermodynamics.
Thermal Engineering can be called Thermodynamics and its applications. If we refer to the syllabus of GATE, then we can find the subject called Thermodynamics and its applications instead of Thermal Engineering.
Let us see what is thermodynamics?
Introduction to Thermodynamics:
Thermodynamics is the branch of mechanical science and sub-division of thermal engineering that deals with the conversion of heat energy into mechanical energy.
What is meant by thermodynamics?
We can observe two words in thermodynamics, they are thermo, and dynamics so, the meaning of the word Thermo is Heat and dynamics means the effect of forces and their motion.
What can we learn from this page?
- Thermal Engineering Syllabus.
- Thermal Engineering basic concepts.
- Thermodynamics Laws.
- Thermodynamic System.
- Thermodynamic Equilibrium.
- Thermal EngineeringTextbooks.
Thermal Engineering Syllabus:
The syllabus is the particulars we read or learn in our semester or academics. As the syllabus will vary according to their University, so I listed out the important syllabus that everyone will follow, and this syllabus is equal to GATE (Graduate Aptitude Test in Engineering). Here I am mentioning the syllabus of Thermodynamics and its applications.
- Thermodynamics 1st Law.
- Thermodynamics 2nd Law.
- Thermodynamics Entropy.
- Analysis of Reversible Process.
- Availability and Irreversibility.
- Power Engineering.
- IC Engines.
- Refrigeration & Psychrometry.
Thermodynamics Laws:
Zeroth Law of Thermodynamics: It states that if two bodies are in thermal equilibrium with the other then they are in thermal equilibrium with each other.
Thermodynamics 1st Law: It states that the energy can neither be created nor destroyed and it can be transferred from one form to another form .It states that heat supplied must be the sum of the work done to the internal energy.
Q = W + E
Thermodynamics 2nd Law: Thermodynamics 2nd law is explained in two statements. They are
According to the Clausius statement, “a self-acting machine in a cyclic process cannot transfer heat from a body at a lower temperature to a body at a higher temperature “.
According to the Kelvin-Planck statement “It is impossible to construct an engine working on a cyclic process, whose sole purpose is to convert heat energy to work”. So sometimes this law is called the Law of Degradation of Energy.
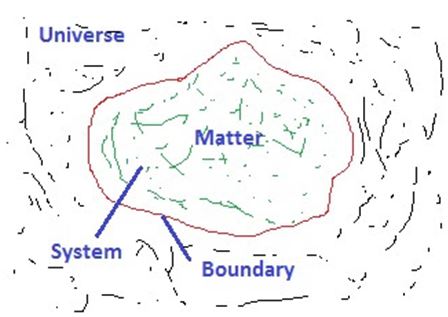
Universe: The combination of the system and the surroundings both is called the Universe.
Thermodynamic System:
The region in the space that contains matter whose behavior is to be investigated is called the Thermodynamic system.
There are three types of Thermodynamic Systems. They are
- Open System
- Closed System
- Isolated System.
- Open System: The system in which both energy and mass transfer takes place between the system and surroundings.
- Closed System: The Closed System in thermodynamics is defined as the transfer of only energy between the system and surroundings and the mass remains constant. Ex: Pressure cooker.
- Isolated System: The system in which the transfer of mass and energy is nil between the System and Surroundings. for example, Ice cubes within the thermal flasks.
Surroundings: The region that does not contain the matter is called the Surroundings.
Boundary: The path of points that separates the system and surroundings is called the Boundary.
Work: Work is defined as the product of force (f)when the distance (d) moved in the direction of x.

The units of force are newtons and units of distance in meters, then the units of work are N-m.
(Where 1 N-M is equal to 1 Joule).
Heat: Heat is defined as the energy transferred without the transfer of mass because of the temperature difference between the system and the surroundings. It is denoted by Q.
Important points:
1. If the heat is flowing into a system is considered Positive.
2. If the heat is flowing out of the system is considered Negative.
Pure Substance: A pure substance is one that has an Invariable chemical composition in all phases.
Examples :
A mixture of liquid water and ice is treated as a two-phase system and is a heterogeneous one.
The chemical composition of the mixture is the same in all phases. it is considered a pure substance.
When air ( A mixture of Oxygen and Nitrogen in its gaseous phase ) when heated gets expanded and when cooled compressed. Then its chemical composition will not alter. But if it partly liquefies its chemical composition will change and will have a higher percentage of nitrogen than when the air in the vapor.
Equilibrium: The Equilibrium is the state where the properties of the system are Uniform and Invariable. The System will not undergo any spontaneous changes when isolated.
It is a state where all Macroscopic properties remain Uniform and Constant.
Thermodynamic Equilibrium:
Thermodynamic Equilibrium can be classified into three types. They are
- Thermal Equilibrium
- Mechanical Equilibrium
- Chemical Equilibrium.
- Thermal Equilibrium: If the two systems are in uniform temperature and having the same temperatures, then it is said to be Thermal Equilibrium.
- Mechanical Equilibrium: When there is no Pressure Gradient within the System, it is said to be Mechanical Equilibrium.
- Chemical Equilibrium: It is the state in which there is no detection change in the chemical composition of a system.
Energy : The energy is defined as the capacity that can able to work. There are two types of energy.They are
- Stored Energy.
- Transition Energy.
- Stored Energy: The energy possessed by the system with its boundaries called Stored Energy. The Stored Energy is of four types. They are
- Potential Energy: The energy possessed by a body or a system for doing work by virtue of its position above the ground level. It is denoted by P.E and the equation is given by

m=mass of the body in Kg
g=gravitaional force
- Kinetic Energy: Kinetic energy is the energy possessed by a body or a system for doing work by virtue of its Mass and Velocity of motion. It is denoted by K.E.

z=distance between the surface and the body in meters
m= mass of the body
v = velocity of the body
- Internal Energy: It is the energy possessed by a body or a system, due to its molecular arrangement and motion of the molecules. It is denoted by U.
- Total energy: It is the sum of the Potential Energy, Kinetic Energy, and Internal Energy. It is denoted by E.
E=P.E+K.E+U
2. Transition Energy: It is the energy possessed by the system which is capable of crossing its boundaries.
Example: Heat, Work, Electrical Energy.
Volume: It is the space occupied by the system. It is denoted by V. The units are cubic meter.
Density: It is the ratio of the mass per unit volume. It is denoted by P (call it a row). The units are kilogram per meter cube

Specific Volume: It is the ratio of the volume per unit mass and it is denoted by v. (or) It is reciprocal to the Density or Mass density. The formula is given by

Enthalpy: It is defined as the sum of the Internal Energy and product of the Pressure, Volume. It is denoted as H and mathematically it is written as
H=U+PV
The units of Enthalpy are Joules or kilo-Joules.
Entropy: It is the thermodynamic property that will increase by the addition of the heat and decrease by the removal of the heat.
( or )
It is inversely proportional to the Temperature T and directly proportional to the Heat Q. It is denoted by S.
Specific Heat: It is defined as the amount of heat required to raise the temperature of a unit mass of any substance through 1 degree. It is denoted by C.

M=mass of the working substance
Q=Heat required
T1 and T2 = Temperatures of two substances.
Displacement or swept volume: The volume covered by the piston while travelling from one dead center to the other is called the Displacement Volume or Swept Volume. The units will be Cubic Centimeter (cc).

Clearance Volume: The volume of the combustion chamber above the piston when it is at the top dead center is the clearance volume. Its units are also expressed in the Cubic Centimeter (cc).
Engine Capacity or Cubic Capacity: The product of displacement volume and the number of cylinders (K) in an engine is called the Engine capacity.
K = Number of cylinders.
Compression Ratio: It is the ratio of the total cylinder volume when the piston is at the bottom dead center to the clearance volume.
Engine power: There are three types of powers in the engine. They are
- Indicated power (IP).
- Brake power (BP).
- Friction power (FP).
- Indicated power (IP): It is defined as the power available at the top of the piston when the combustion takes place. It is the product of the mass flow rate of air and the network done in the engine.

Pim = mean effective pressure (N/m2)
Vs = volume of the engine = L * A ( m3 )
N= speed in revolutions per minute.
n = number of power strokes per minute.
K = number of cylinders.
- Brake Power (B.P): It is the rotational force available at the delivery point, at the engine crankshaft, and the power of it is called the Brake Power (B.P).
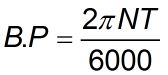
- Frictional power (FP): It is the difference between the Indicated power (I.P) and the Brake power (B.P)
F.P = I.P – B.P
Calorific Value: It is the thermal energy obtained per unit quantity of the fuel. Every fuel has its calorific value according to its combustion capacity. It is expressed in terms of KJ/Kg.
The calorific value of Petrol is 48000 KJ/Kg.
The calorific value of Diesel is 44800 KJ/Kg.
It is the main factor and the parameter which should be considered in selecting the fuel.
Efficiency: The performance of an engine is termed as efficiency. The types of efficiencies are as follows:
- Indicated Thermal Efficiency: It is the ratio of the Indicated power to the input fuel or mass of the fuel. It is expressed as

It is expressed in percentage ( %).
- Brake Thermal Efficiency: It is the ratio of the brake power to the fuel energy or mass of fuel. It expressed as .

- Mechanical Efficiency: The ratio of Brake power and the Indicated power. It can be expressed as nm.

Thermal Engineering Textbooks (for reference):
Students need to refer to the textbooks to grab the knowledge that is not even found in the classroom teaching. When the student starts referring to the textbooks then the seed of knowledge will start growing in oneself. So, do not stick to the classroom notes but also try to refer to the textbooks that increase the knowledge.
Here I am mentioning a few references, please go through them.
- Thermal Engineering by P K Nag.
- Engineering Thermodynamics by Rajput.
- Thermal Engineering by Ganeshan.
- Thermal Engineering Textbook by R S Khurmi.
Note: Please refer to the syllabus in each textbook before you refer to or purchase